Molecular Cations and Anions
Molecules can also lose or gain electrons to become cations or anions. For example, the NO3 molecule will gain an electron to form the nitrate anion.
If you count up all of the electrons you'll find that all of the atoms feel like neon.
Get the free 'Lewis structure' widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram Alpha. The Na + cation and the Cl-anion are held together by electrostatic or ionic bonds.There is no sharing in ionic bonding. The anion takes the electron for itself and the cation is happy to get rid of its electron. The ions in ionic compounds are arranged in three-dimensional structures. Lewis Electron Dot Structure Calculator. What is the lewis dot structure for ozone? Chemistry stack exchange electron (lewis) structures pages 1 4 flip pdf download fliphtml5 why hcooh written like bottom but not top? Doesn t top satisfy octet rule?: chemhelp formation of kcl with an structure? Quora diagram chemical bonds full version hd quality diagramtonyb nowroma it. Lewis Dot Structures-Covalent: Metallic Bonds: Like Dissolves Like. TED ED Dissolving Video: 70 Lewis Dot Structures Videos (AP) Valence Shell Electron Pair Repulsion VSEPR(Shapes) Percent Composition by Mass. Lewis Dot Structures -Ionic: Molecular polarity: Percent Water in a Hydrate: Properties of Ionic Compounds: Intermolecular Force. A step-by-step explanation of how to draw the Li2O Lewis Dot Structure.For Li2O we have an ionic compound and we need to take that into account when we draw.
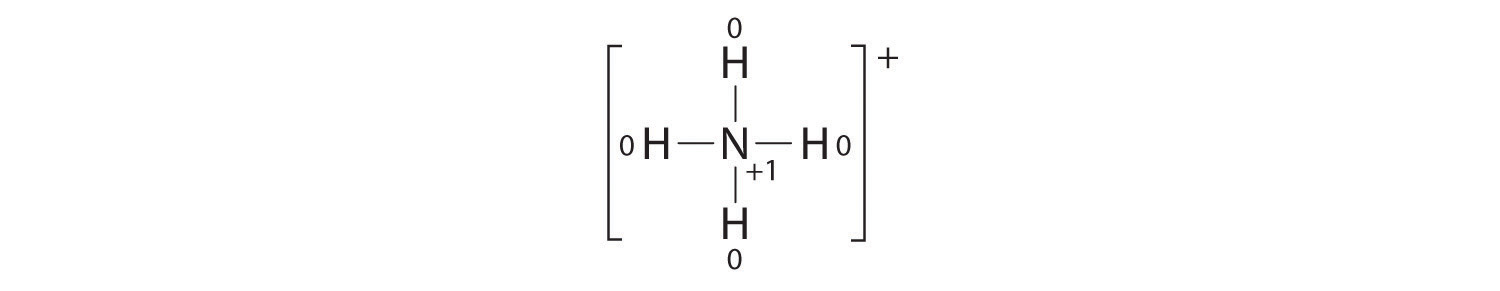
Here is the ammonium ion, an example of a molecular cation.
The ammonium ion has given up an electron to become a cation.
Ionic Bonds
Ionic bonds are generally formed when you bring atoms which really want to lose electrons together with atoms which really want to gain electrons.
The Na+ cation and the Cl- anion are held together by electrostatic or ionic bonds. There is no sharing in ionic bonding. The anion takes the electron for itself and the cation is happy to get rid of its electron. The ions in ionic compounds are arranged in three-dimensional structures. There are no discrete molecules of NaCl. We can only write an empirical formula of NaCl.
Here are some other examples.
| NH4Cl : | here NH4+ | = cation |
| here Cl- | = anion | |
| BaCl2 : | here Ba2+ | = cation |
| here Cl- | = anion |
All substances are electrically neutral. We can use this fact to obtain the chemical formula of an ionic compound.
| Ba2+ | and | SO42- | form | BaSO4 |
| Na+ | and | S2- | form | Na2S |
Notice that in Na2S, two sodium cations were needed to balance the -2 charge of S2-, making things electrically neutral.